Nanotechnology Breakthrough May Improve Drug Delivery
Columbia Engineering researchers have developed a technique to isolate a single water molecule inside a buckyball, or C60, and to drive motion of the so-called “big” nonpolar ball through the encapsulated “small” polar H2O molecule, a controlling transport mechanism in a nanochannel under an external electric field. They expect this method will lead to an array of new applications, including effective ways to control drug delivery and to assemble C60-based functional 3D structures at the nanoscale level, as well as expanding our understanding of single molecule properties. The study was published as a “Physics Focus” in the April 12 issue of Physical Review Letters.
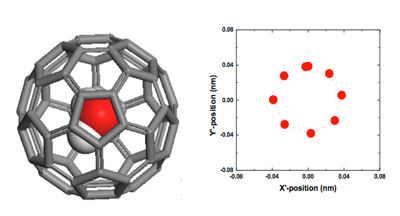
The structure of a single water molecule imprisoned inside a fullerene C60 at equilibrium (left), and projection trajectories of the encapsulated H2O molecule (mass center) within one period for steady-state transport of H2O@C60 under an electric intensity of 0.05 V/Å(right).
“Buckyballs, more formally known as Buckminsterfullerenes, or fullerenes, are spherical, hollow molecular structures made of 60 carbon atoms, with the size of ~1 nm—6,000-8,000 times smaller than a regular red blood cell—and, because of their highly symmetrical structure, very hydrophobic core, covalentnonpolar bonds, and more importantly, relatively non-toxicity to the human body, they are a perfect container for drug molecules,” explains Xi Chen, associate professor of earth and environmental engineering, who led the research. He and his team believe their work is the first attempt to manipulate a nonpolar molecule (C60) or structure by an inserted polar molecule (H2O).
Chen says his findings may open a new way of controlling and delivering a nonpolar “big” molecule like C60 through the encapsulated “small” polar molecule like H2O. This could lead to important applications in nanotech and biotech areas, including drug delivery where researchers can “imprison” the polar drug molecules inside a hollow structure and then guide them to their targets.
And, from a fundamental point of view, he hopes that the isolated, encapsulated single molecule, like the H2O one in his study, will provide an important platform for revealing and probing inherent characteristics of a single molecule, free from its outside environment.
“The important role of hydrogen bonds in the properties of water, like surface tension and viscosity, and the precise interactions between a single water molecule and hydrogen bonds, are still unclear,” Chen notes, “so our new technique to isolate a single water molecule free from any hydrogen bonds provides an opportunity for answering these questions.”
Since the discovery of C60 in the 1980s, scientists have been trying to solve the challenge of controlling a single C60. Several mechanical strategies involving AFM (atomic force microscopy) have been developed, but these are costly and time-intensive. The ability to drive a single C60 through a simple external force field, such as an electrical or magnetic field, would be a major step forward.
In the Columbia Engineering study, the researchers found that, when they encapsulated a polar molecule within a nonpolar fullerene, they could use an external electrical field to transport the molecule@fullerene structures to desired positions and adjust the transport velocity so that both delivery direction and time were controllable. Chen’s team came up with the idea a year ago, and confirmed their surprising results through extensive atomistic simulations.
Chen plans to explore more properties of the H2O@C60 molecule and other similar structures, and to continue probing the interaction and communication of the encapsulated single water molecule with its surroundings. “Studying the communication of an imprisoned single water molecule with its outside environment such as adjacent molecules,” he adds, “is like learning how a person sitting inside a room makes connections with friends outside, selectively on demand (i.e. with control) or randomly (without control) through, say, over the phone.”
This research was funded by the National Science Foundation and DARPA (Defense Advanced Research Projects Agency).